The world of diagnostics is evolving rapidly, driven by the growing need for personalized healthcare solutions. For decades, qPCR has been the backbone of diagnostic testing, offering unmatched speed, sensitivity, and efficiency for routine applications. However, as the complexity of diseases like cancer, genetic disorders, and emerging infectious diseases increases, so does the demand for technologies that can deliver deeper, broader insights.
In this blog, we explore how qPCR and NGS each play a vital role in molecular diagnostics, their unique strengths and limitations, and how combining these technologies can create a powerful, holistic approach to tackling complex diagnostic challenges. Whether identifying known disease markers quickly or uncovering novel variants for personalized treatment, knowing when to use each technology and how they complement each other is key to advancing diagnostics.
The Growing Demand for Personalized Diagnostics is Driving Innovation Beyond qPCR
Over the next four years, the personalized healthcare market is projected to grow at a CAGR of 10.8%, reaching nearly $590 billion by 2028.1,2
This growth is driven by the rising demand for personalized therapeutics and diagnostics as healthcare increasingly adopts tailored, patient-centered approaches. By integrating genetic, environmental, and lifestyle data, personalized medicine enables early detection and customized treatments tailored to a patient’s unique genetic profile. This approach is particularly vital for managing complex conditions such as cancer, autoimmune disorders, and genetic diseases, which can vary significantly between individuals.
While qPCR remains highly efficient and cost-effective for routine diagnostics, such as infectious disease testing, its role in personalized medicine is limited by its inability to capture the full genetic complexity of a patient’s genome. Designed to detect specific, pre-identified genetic markers using probes or primers, qPCR is limited in its ability to uncover the broad spectrum of mutations and genomic variations present in diseases like cancer. Identifying the unique genetic fingerprint of a disease is crucial for individualized treatment planning, as these mutations often play a key role in influencing therapeutic responses. To address these challenges, next-generation sequencing (NGS) is increasingly favored for its ability to analyze entire genomes or exomes, uncovering both known and novel variations that are essential for tailoring therapies to each patient’s unique genetic profile.
Next-Generation Sequencing
Next-generation sequencing (NGS) was first applied in diagnostics around the late 2000s3, providing transformative capabilities beyond what PCR-based and traditional sequencing methods could offer. Its ability to generate vast amounts of data from a single DNA sample provides exceptional analytical power, enabling high-throughput and comprehensive genomic analysis.
Unlike qPCR, which relies on probes or primers tailored to known variants, NGS reads large sections of genetic material, allowing for the detection of both known and novel variants in a single assay. It enables comprehensive analysis of entire genomes, exomes, or specific gene panels without needing prior knowledge of target sequences. This adaptability makes it suitable for a wide range of applications, including oncology, infectious disease monitoring, and hereditary disease testing.
A key factor driving the increased use of NGS is the reduced cost of sequencing, which has significantly enhanced its accessibility. Advances in sequencing technologies, combined with AI integration, have also improved its accuracy, efficiency, automation, and portability. These advancements have accelerated the pace of genomic analysis and enabled broader applications, such as precision medicine, where treatments are increasingly tailored to the genetic makeup of individual patients.4 Overall, the shift towards more accessible and rapid genomic testing has been essential in fueling the growth of precision medicine, especially in oncology, where timely genetic insights can dramatically influence treatment choices.
Choosing Between qPCR and NGS for Diagnostic Applications
When deciding between qPCR and NGS for diagnostic applications, it is essential to weigh the unique strengths of each technology. qPCR excels in scenarios where rapid, sensitive detection of specific, known genetic markers is required. Its speed and cost-effectiveness make it the preferred choice for routine testing, such as identifying pathogens in infectious diseases or confirming the presence of well-characterized mutations in hereditary conditions.
However, qPCR’s reliance on pre-designed probes or primers limits its ability to identify unknown or novel variants. This constraint is particularly limiting in complex scenarios such as oncology or emerging infectious diseases, where the dynamic and intricate genetic landscape demands a broader, more comprehensive approach.
NGS bridges this gap. NGS uncovers known and novel variants in a single assay by sequencing entire genomes, exomes, or targeted gene panels. This adaptability makes it invaluable for applications such as:
- Infectious Disease Surveillance: NGS sequences entire viral genomes, enabling the detection of emerging variants and aiding the public health response to evolving outbreaks.
- Cancer Diagnostics: Tumors often exhibit a complex array of mutations, including rare and novel variants. Comprehensive genomic profiling through NGS helps identify actionable mutations that guide precision therapies.
- Genetic Disorder Diagnosis: Hereditary conditions often involve rare or unique mutations. NGS provides the depth needed to uncover the full spectrum of variants associated with these disorders.
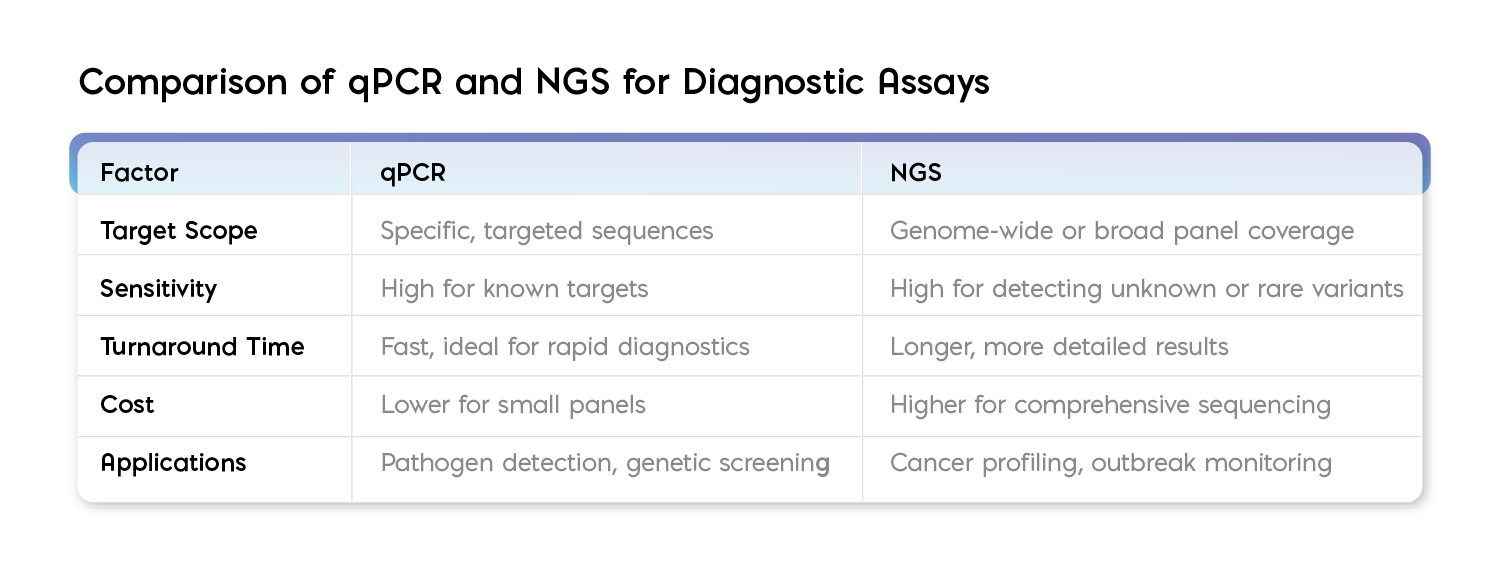
In summary, while qPCR delivers quicker results and can accelerate the initiation of targeted therapy, NGS, although slower, provides richer insights, making it the technology of choice for applications where genetic complexity demands a deeper understanding. Together, these technologies offer complementary strengths that can be leveraged to create more robust diagnostic workflows.
The Potential of a Holistic Technological Approach to Diagnostic Testing
The diagnostic landscape is moving toward a hybrid approach, where qPCR and NGS are integrated to maximize the strengths of each technology. Combining the speed and precision of qPCR with the comprehensive insights of NGS can significantly enhance diagnostic accuracy and efficiency.
For example:
- Stepwise Testing: Start with qPCR for rapid screening of known mutations or pathogens. When results are inconclusive or more information is needed, follow up with NGS to explore novel variants or broader genomic regions.

- Layered Surveillance: In infectious disease monitoring, qPCR is able to track specific variants of concern in real-time, while periodic NGS can provide a validation layer by sequencing the full pathogen genome. This approach was successfully used during the COVID-19 pandemic to track and monitor emerging variants5.
- Comprehensive Oncology Workflows: For cancer diagnostics, qPCR can serve as a first-pass tool to detect known mutations, while NGS identifies rare or complex mutations that drive personalized treatment strategies. This dual-technology model offers flexibility and scalability, allowing laboratories to tailor their workflows to the demands of individual cases. By leveraging qPCR for its speed and cost-effectiveness and NGS for its depth and adaptability, diagnostic providers can create a future-ready solution that addresses both routine and complex diagnostic needs.
Meridian is uniquely positioned to support this hybrid approach. With our range of enzymes and master mixes for qPCR and NGS, we provide solutions that enable seamless integration of these technologies. From lyo-ready master mixes for ambient-temperature stability to glycerol-free formulations for enhanced performance, our products are designed to support diagnostics at every level.
Conclusion: A Future of Complementary Diagnostics
As diagnostics evolve, the integration of qPCR and NGS is becoming essential to meet the growing demand for precision and personalized medicine. While qPCR remains the gold standard for rapid and targeted testing, NGS offers unparalleled depth and adaptability for uncovering novel and complex genetic information. Together, these technologies provide a pathway to more efficient, accurate, and comprehensive diagnostics, enabling better patient outcomes. This hybrid approach also enhances diagnostic workflows, supporting the growing demand for precision medicine and personalized healthcare solutions.
Meridian is at the forefront of this transformation, offering a portfolio of innovative solutions designed to empower diagnostic developers. From lyo-ready, ambient-temperature stable reagents to high-concentration, glycerol-free enzymes tailored for high-throughput workflows, our products are engineered to enhance performance, streamline operations, and support the most advanced diagnostic applications. Additionally, Meridian’s master mixes are optimized for multiplexing qPCR assays, enabling simultaneous detection of multiple targets with high sensitivity and specificity. Some of our leading products include:
- dUTP master mixes formulated with dUTP to prevent carryover contamination in high-throughput settings, when working with high-sensitivity applications, or reusing equipment between reactions
- Lyo-Ready and Air-Dryable™ master mixes designed for creating ambient-temperature stable assays
- High-concentration and glycerol-free enzymes for qPCR and NGS that are compatible with lyophilization
- Glycerol-free NGS library preparation kit and enzymes that aim to reduce the cost of NGS testing and improve NGS portability
Learn more here or contact us today to discuss how our tailored solutions can optimize your molecular diagnostics workflows.
References:
1. Global Market Estimates. (2023). Personalized healthcare market analysis: Size & forecasts by region, product, and end-user, 2023-2028. Retrieved from https://www.globalmarketestimates.com
2. Markets and Markets. (2024). Personalized medicine market by product, application, end-user, & region – global forecast to 2028. Retrieved from https://www.marketsandmarkets.com
3. Drug Target Review. (2015, January 31). How next-generation sequencing came to be: A brief history. Drug Target Review. Retrieved from https://www.drugtargetreview.com
4. Clinical Lab Products. (2024). Next-generation sequencing: Driving innovation in precision medicine. Clinical Lab Products. Retrieved from https://www.clpmag.com
5. Carattini, Y. L., et al. (2023). Combined use of RT-qPCR and NGS for identification and surveillance of SARS-CoV-2 variants of concern in residual clinical laboratory samples in Miami-Dade County, Florida. Viruses, 15(3), 593. https://doi.org/10.3390/v15030593