In recent years, point-of-care testing (POCT) and next-generation sequencing (NGS) have undergone remarkable advancements, establishing themselves as fundamental tools in modern healthcare. Their use has traditionally been viewed as somewhat opposing: POCT prioritizes speed and portability, whereas NGS is laboratory-based, labor-intensive and slow. However, building on the successful integration of PCR with POCT, it anticipates a similar synergy between POCT and NGS, driving the next wave of innovation in rapid and precise diagnostics. Technological advancements are blurring the lines between NGS and POCT, signaling a promising integration of speed and accuracy, which has become particularly evident in microfluidic devices for diagnostic testing.
Mini Marvels: POCT Microfluidic Devices

The traditional diagnostic method RT-qPCR is highly integral to the field of clinical diagnostics and research; however, it has limitations in scalability and ease of use. The COVID-19 pandemic not only highlighted its insufficiencies especially in resource-limited settings, but opened the door to newer, more adaptable platforms such as NGS and POCT.
The Value of NGS Testing
The power of NGS lies in its ability to unlock previously inaccessible information for researchers and clinicians. The technology can deliver comprehensive genomic data enabling the analysis of hundreds of biomarkers in a single assay, providing detailed genetic insights that help guide critical health decisions. Traditionally, the bulk and complexity of NGS equipment confined it to laboratory settings. However, continuous improvements in NGS technology, including increased speed, portability, accuracy, and reduced costs, have made it more accessible and convenient.
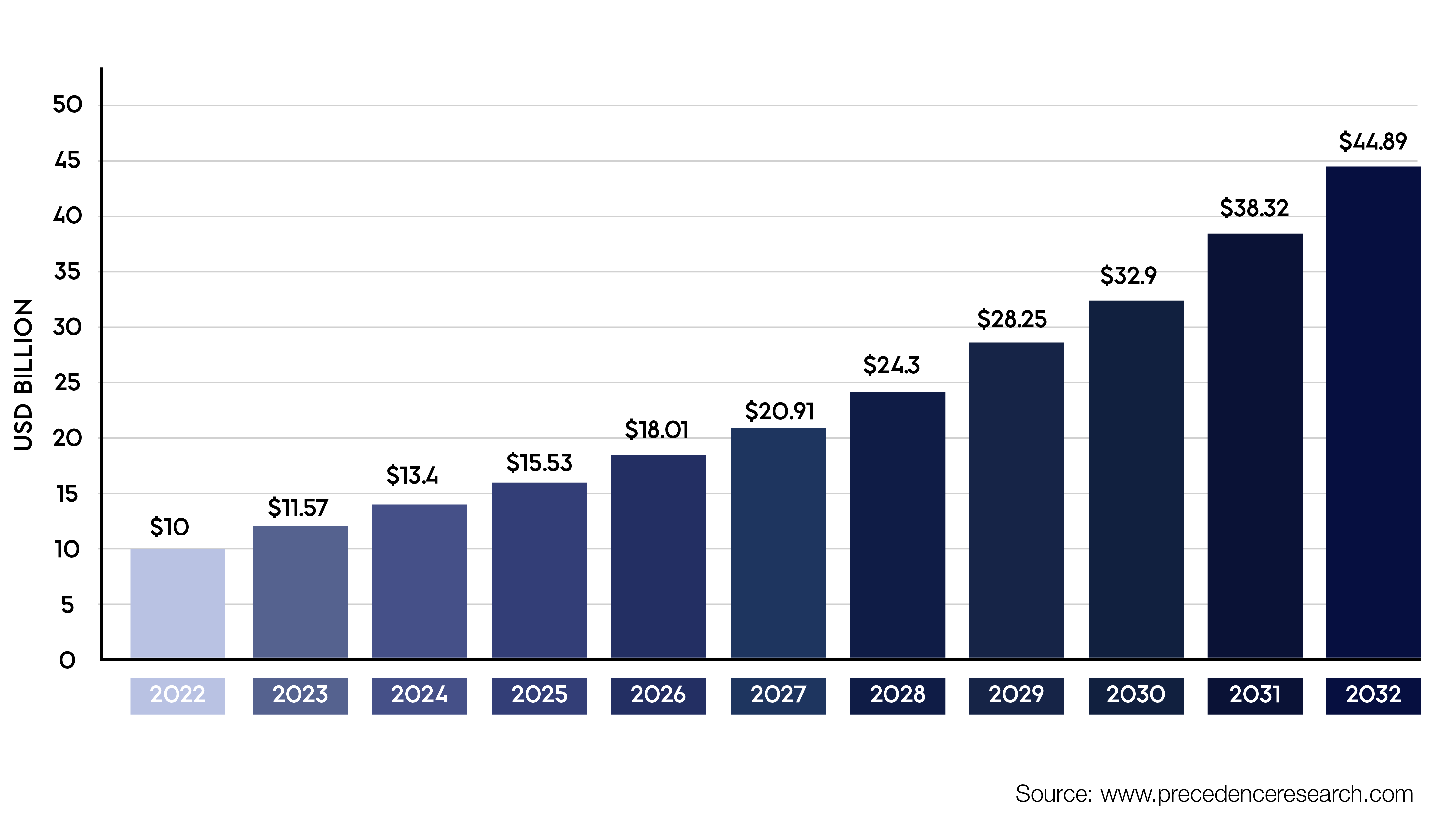
Despite the relatively higher cost of NGS compared to other molecular diagnostic methods, NGS is expanding in the healthcare industry, especially in areas where detailed genetic insights can improve treatment and care, such as in non-invasive prenatal testing (NIPT), oncology, pharmacogenomics. Overall, the application of NGS into clinical testing and disease diagnostics is increasing at a staggering rate and its market value is projected to grow tenfold by 2032, increasing from $5.9 billion to $44 billion globally1.
Growth in NGS Market 2022 to 2032
As modern NGS platforms become more compact and user-friendly, their integration into clinical workflows with high-throughput sequencing will enable the simultaneous analysis of multiple samples increasing efficiency, particularly in large-scale studies and clinical trials. With the growing demand for advanced healthcare, NGS is poised to transform patient care, delivering critical insights that enhance outcomes and deepen our understanding of genetics.
The Surge in POCT Innovation
POCT diagnostics have become invaluable for immediate diagnosis, significantly improving patient outcomes. Like NGS, the POCT market has surged due to technological advancements. Innovations utilizing cutting-edge molecular diagnostic techniques such as isothermal amplification methods, have made POCT more user-friendly and robust.
Microfluidic chips have notably advanced POCT by enabling precise control and manipulation of small fluid volumes on a microscale. These chips capture and analyze a wide range of biological samples, including blood, saliva, urine, and other bodily fluids. They are particularly effective at capturing:
- Cells, such as white blood cells, red blood cells, and circulating tumor cells (CTCs).
- Biomolecules, including proteins, nucleic acids (DNA, RNA), enzymes, and antibodies.
- Pathogens, such as bacteria, viruses, and other microorganisms.
- Chemical Analytes, including glucose, electrolytes, and other small molecules.
Microfluidic chips, designed to capture CTCs and analyze cell-free DNA (cfDNA), represent a major advancement in POCT especially in oncology. These chips are beneficial because they enable precise, real-time analysis of biological samples with minimal sample volumes, making them ideal for various applications, from RNA sequencing to mutation analysis, without the need for complex laboratory setups. This capability has propelled microfluidic chips as a powerful tool in expanding POC diagnostics, bringing critical testing directly to the patient.
However, while microfluidics excels in handling small sample volumes, certain applications, such as cancer biomarker detection, require larger volumes to process low-abundance analytes in complex biological samples. Balancing the benefits of miniaturization with the need for diagnostic sensitivity and accuracy remains a technological challenge that must be addressed to unlock the full potential of microfluidics in clinical diagnostics.
Integrating NGS and POCT: Opportunities and Challenges
As both POCT and NGS continue to evolve technologically, their integration promises to combine complementary strengths: comprehensive genomic analysis with rapid response capabilities. The greatest benefit is that clinicians could achieve in-depth genetic analysis with quick turnaround times, leading to faster and more accurate disease detection and management2.
Unlocking the Potential of NGS for Point-of-Care Applications: What’s Needed to Bridge the Gap
Such a powerful synergy has the potential to transform patient care, particularly in emergency settings where timely information is crucial, and in resource-limited environments, where conventional diagnostic methods may fall short. By providing detailed actionable data swiftly and efficiently, this integration opens avenues for more precise, personalized treatment plans. This could enhance overall healthcare outcomes through effective monitoring of individual responses to therapy and early detection of prognostic or diagnostic biomarkers at any stage of a disease.
However, integrating NGS with POCT is not without its challenges. NGS tests typically involve temperature-sensitive reagents that require cold chain shipping and storage, while POCT devices require robust operation at ambient temperature and in diverse environments to be effective. A major hurdle is ensuring NGS reagents can remain stable at ambient temperatures and are compatible with the miniaturized components of a microfluidic device. Additionally, miniaturization must balance the need for compactness with the requirement to maintain high performance and reliability, ensuring accurate operation despite the device’s small size and varying conditions.
Better data interpretation is also essential. Combining detailed genomic data with rapid test results requires robust bioinformatics tools to accurately interpret the data for effective clinical decision-making.
The Road Ahead
Innovations in NGS testing and POCT devices continue to push the boundaries of diagnostics. The pandemic catalyzed rapid advancements in molecular diagnostics, potentially serving as a blueprint for tackling future infectious diseases. Moreover, the emergence of compact devices is making sequencing more convenient and portable, bringing the widespread integration of advanced NGS and POCT technologies closer to reality.
As AI is incorporated into NGS applications, diagnostics will become not only more precise but also faster. In the near future, real-time genomic data analysis could assist clinicians in making swift treatment decisions. AI’s ability to detect complex patterns significantly enhances NGS, increasing its potential as a ground-breaking tool for cancer detection and treatment.
The advent of portable sequencing devices marks a significant step towards the democratization and accessibility of sequencing. When combined with POCT, it enables immediate, in-depth diagnostics – such as accessing multiple biomarkers from a single test – along with comprehensive genomic information, even in remote locations. The synergy of NGS’s accuracy and POCT’s speed can redefine rapid diagnostic testing, expanding its menu and making it more personalized, precise, and timely.
From Concept to Reality: Meridian Paving the Way for NGS/POCT Integration
Transforming new products from proof-of-concept design into tangible, scalable products is challenging, especially for innovative integrated NGS/POCT devices. Specifically, designing microfluidic devices requires advanced technical expertise and material compatibility.
Meridian’s new glycerol-free NGS library preparation enzymes aim to reduce the cost of NGS testing and improve NGS portability, enabling researchers to perform sequencing in various environments, such as resource-limited locations or point-of-care settings. The advantages of Meridian’s NGS solutions include:
- Glycerol-Free: Compatible with lyophilization, stable at room temperature for at least 12 months while maintaining complete enzyme activity, avoiding cold chain transport and reducing costs
- High-Concentration Formula: Ideal for miniaturized POCT devices, providing greater flexibility in reaction configuration and maximizing sample input volume, thereby enhancing detection sensitivity
- Simplified Steps: Features specially optimized reaction buffers that combine end repair and A-tailing in a single step, streamlining the operation process.
With the ongoing evolution of NGS and POCT, fueled by technological advancements and AI integration, the future promises rapid, portable, and highly accurate diagnostics. As these fields continue to converge, the potential for improved patient outcomes and more efficient healthcare solutions becomes increasingly tangible.
To learn more, visit our website
References:
1. Research, P. (2023, October 30).Next Generation Sequencing Market Size, growth,Share, and Trends 2024 to 2034. https://www.precedenceresearch.com/next-generation-sequencing-market
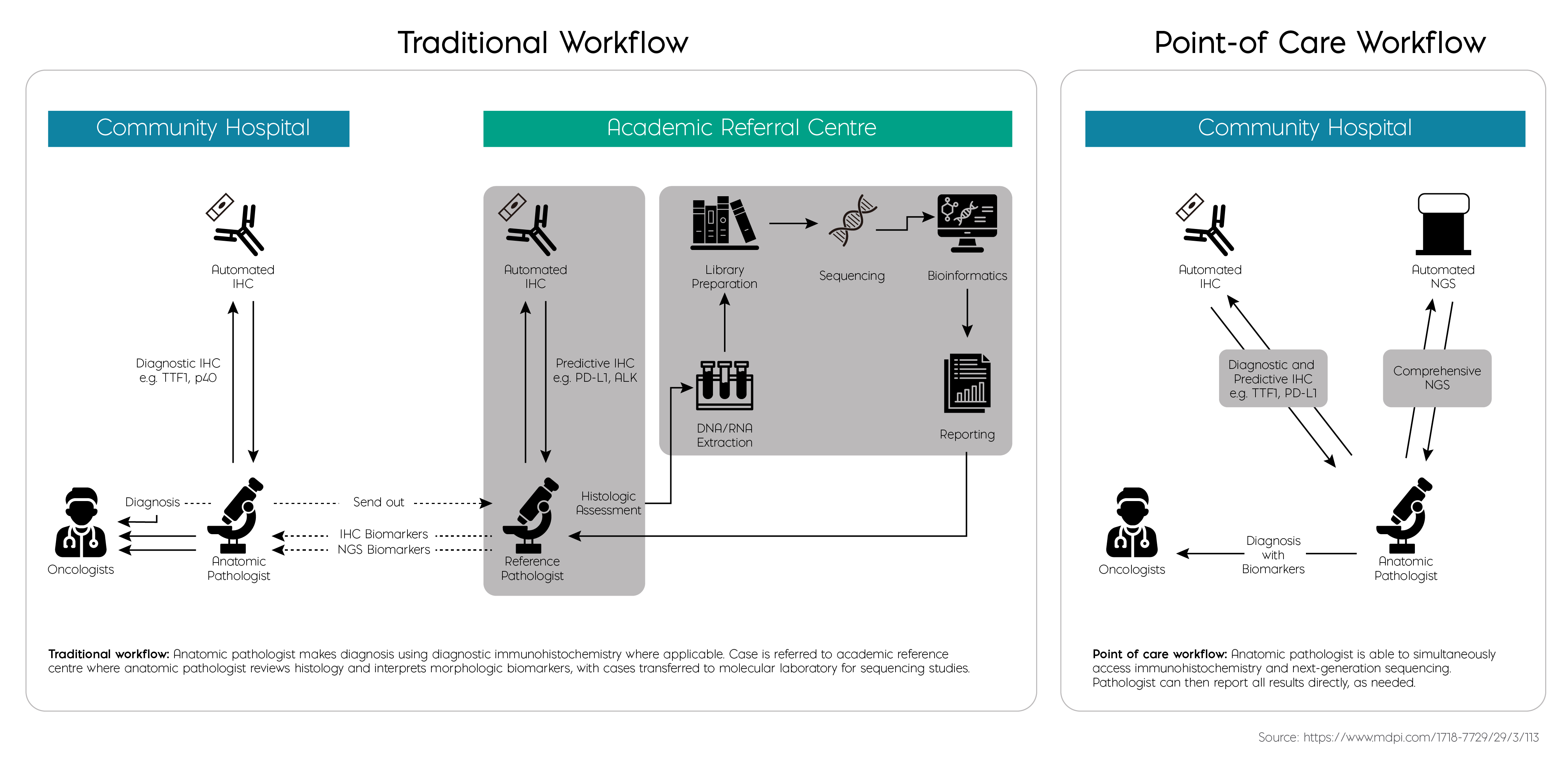
2. Sheffield, B. S., Beharry, A., Diep, J., Perdrizet, K., Iafolla, M. a. J., Raskin, W., Dudani, S., Brett, M. A., Starova, B., Olsen, B., & Cheema, P. K. (2022). Point of Care Molecular testing: Community-Based Rapid Next-Generation Sequencing to support cancer care. Current Oncology, 29(3), 1326–1334.https://www.mdpi.com/1718-7729/29/3/113